Introduction
Chronic bacterial prostatitis (CBP) is a persistent and often debilitating condition that significantly impacts men’s quality of life. Traditional antibiotic therapies frequently fall short due to rising antibiotic resistance, leading researchers to explore alternative treatments. One groundbreaking approach is bacteriophage therapy, which harnesses viruses that target and destroy bacteria. A recent case study published in the International Journal of Clinical Virology highlights the successful use of bacteriophage-antibiotic combination therapy in treating CBP, demonstrating its potential as an effective alternative to conventional treatments. Visit HSPIOA for more cutting-edge research on infectious disease treatments.
Chronic Bacterial Prostatitis and the Challenge of Antibiotic Resistance
CBP is a form of prostatitis characterized by recurrent urinary tract infections (UTIs), pelvic discomfort, and persistent bacterial colonization of the prostate. Escherichia coli (E. coli) is the most common pathogen responsible for CBP, often exhibiting multi-drug resistance (MDR) due to biofilm formation, making standard antibiotic regimens less effective.
Traditional treatments involve prolonged courses of fluoroquinolones or trimethoprim–sulfamethoxazole, but recurrence rates remain high, emphasizing the need for innovative solutions. This study explores the role of bacteriophage-antibiotic synergy in overcoming these limitations.
Case Study: Successful Resolution of CBP with Phage Therapy
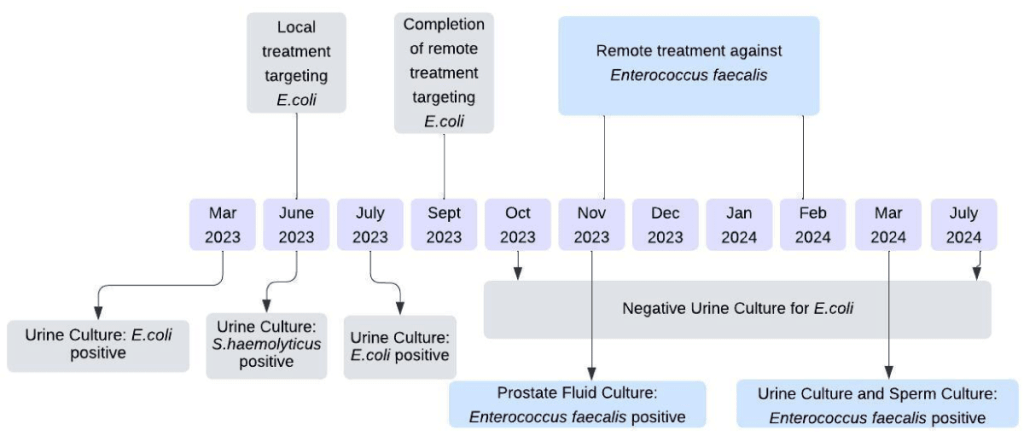
A 65-year-old patient suffering from chronic E. coli-induced prostatitis had undergone multiple unsuccessful antibiotic treatments before seeking alternative therapy at the Eliava Phage Therapy Center in Tbilisi, Georgia. Key aspects of his treatment included:
- Customized bacteriophage therapy developed specifically to target the MDR strain of E. coli.
- Phage-antibiotic combination treatment, leveraging the synergy between phages and Bactrim (sulfamethoxazole/trimethoprim).
- A structured regimen involving oral and rectal phage applications over several treatment cycles.
Following 35 days of phage-antibiotic therapy, urine culture tests showed a significant reduction in bacterial load, ultimately leading to complete bacterial eradication. Notably, the patient experienced symptom resolution, with no adverse reactions to phage therapy.
Why Bacteriophage Therapy Works
Bacteriophages (phages) are viruses that specifically infect and lyse bacteria, offering several advantages over antibiotics:
- Targeted action: Unlike broad-spectrum antibiotics, phages attack only the disease-causing bacteria, preserving beneficial microbiota.
- Biofilm penetration: Phages can effectively break down bacterial biofilms, a key factor in chronic infections.
- Lower resistance risk: Bacteriophage therapy can reduce the likelihood of antibiotic resistance development when used in synergy with antibiotics.
The success of phage therapy for CBP supports growing interest in personalized bacteriophage treatments, particularly in cases where standard antibiotics fail.
Global Perspectives on Bacteriophage Therapy
Leading organizations such as the Pan American Health Organization (PAHO) recognize the urgent need for novel antimicrobial strategies in combating resistant bacterial infections. Clinical trials continue to explore phage therapy’s potential in urological infections, paving the way for broader medical adoption.
Future of Phage Therapy in Prostatitis Treatment
As antibiotic resistance continues to rise, phage-antibiotic synergy offers a viable, personalized treatment option for patients with recurrent bacterial prostatitis. The findings from this study reinforce the importance of continued clinical research and development in bacteriophage applications.
Image Representation of Bacteriophage Therapy Timeline
Read the full study at https://doi.com/10.29328/journal.ijcv.1001059 for a detailed analysis of this innovative treatment approach.
Join the Conversation!
What are your thoughts on bacteriophage therapy for chronic infections? Share your insights in the comments below, and explore more groundbreaking research at HSPIOA!
Disclaimer: This content is generated using AI assistance and should be reviewed for accuracy and compliance before considering this article and its contents as a reference. Any mishaps or grievances raised due to the reusing of this material will not be handled by the author of this article


Leave a comment